Chemistry. D and F Block - 3 Session Session Objectives 1.f-Block elements 2.Introduction to lanthanides 3.Oxidation state 4.lanthanide contraction 5.Chemical. - ppt download

Comparison of Ti, Zr, and Hf as Cations for Metallocene-Catalyzed Olefin Polymerization | Organometallics

Comparison of the atomic radii and electronegativity of the elements... | Download Scientific Diagram

Change in atomic radius as a function of the period in the periodic... | Download Scientific Diagram
SOLVED:Zr and Hf have almost equal atomic and ionic radii because: (a) of diagonal relationship (b) of lanthanide contraction (c) of actinide contraction (d) hoth ho ane e

SOLVED: The lanthanide contraction is responsible for the fact that (a) Zr and Y have about the same radius (b) Zr and Zn have the same oxidation state (c) Zr and Hf

Predicting Nitrogen‐Based Families of Compounds: Transition‐Metal Guanidinates TCN3 (T=V, Nb, Ta) and Ortho‐Nitrido Carbonates T′2CN4 (T′=Ti, Zr, Hf) - Luo - 2021 - Angewandte Chemie International Edition - Wiley Online Library
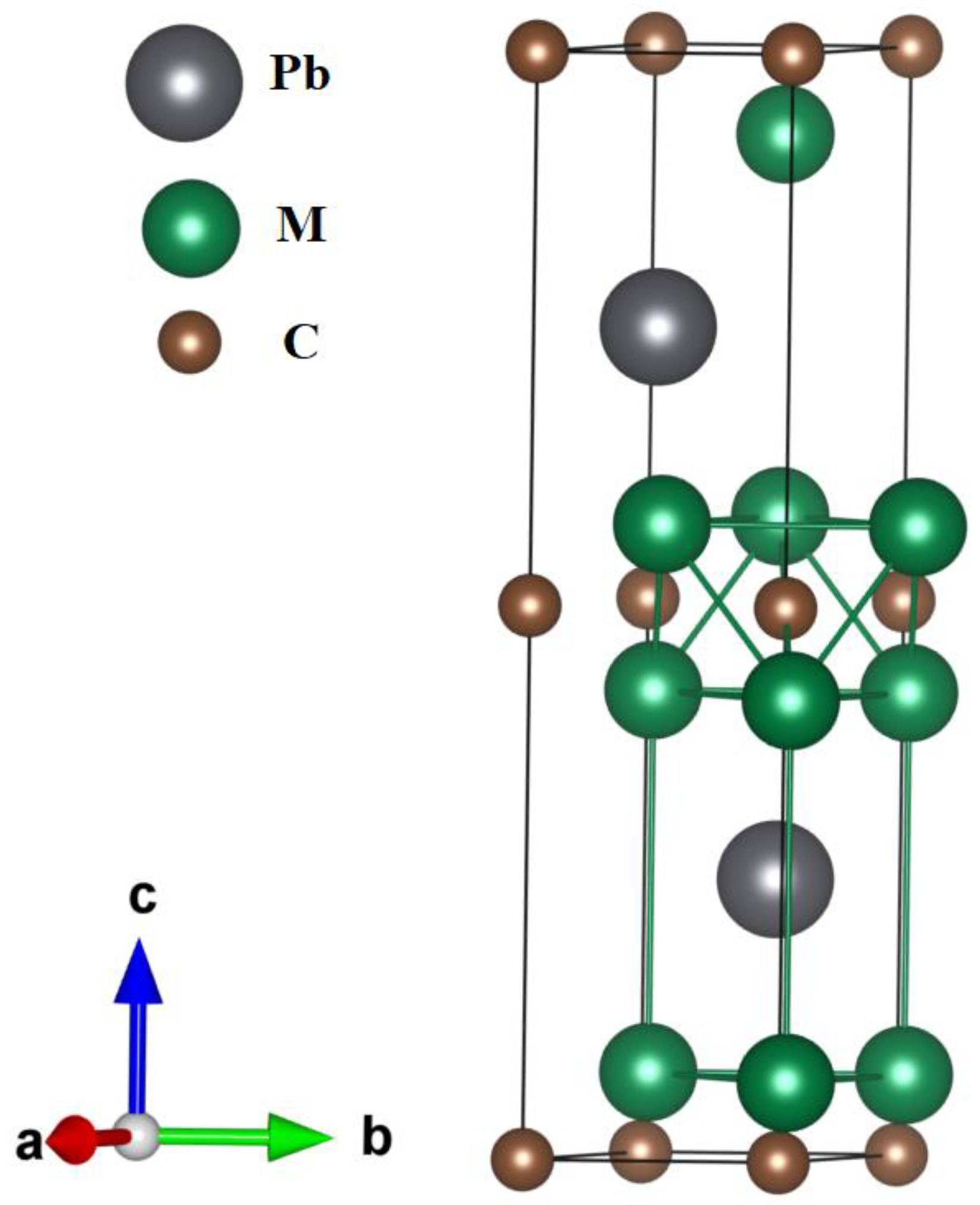
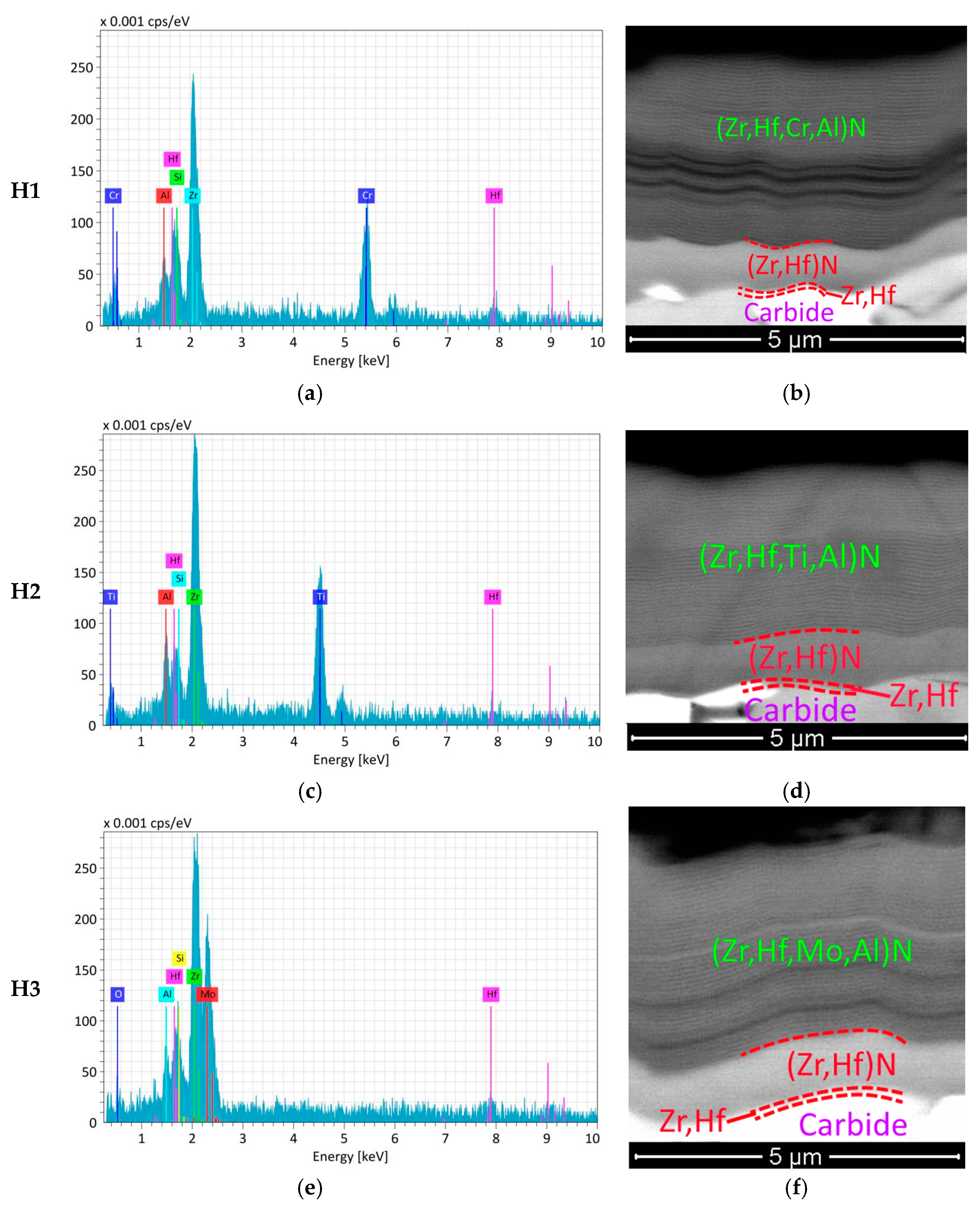
Crystals | Free Full-Text | Physical Properties Investigations of Ternary-Layered Carbides M2PbC (M = Ti, Zr and Hf): First-Principles Calculations